VITAL 2.0
Incidental contamination with allergenic substances as a result of "cross-contact" is a frequent issue in food-processing plants. Potential causes include the use of contaminated raw ingredients as well as allergenic substances carried over in the production process, e. g. due to insufficient cleaning procedures or when a joint production line is used for foods containing allergens and for allergen-free foods. As a precautionary measure and for product liability reasons, food manufacturers label their foods with declarations such as "may contain" or "contains traces of". The increasing use of such declarations, however, restricts the choice of foods for allergy sufferers.
Below you will find a general introduction to the VITAL concept. For more information, please see http://www.allergenbureau.net/vital/vital/ or the following links:
Voluntary declaration
In 2007, several international food producers and the Australian Food and Grocery Council (AFGC) developed the VITAL concept ("Voluntary Incidental Trace Allergen Labelling"). This concept is a standardised approach for the detection and threshold-based declaration of inadvertent traces of allergens in foodstuffs. The concept is already being applied by several food manufacturers in Australia.
A revised VITAL concept - commissioned by the Allergen Bureau - has been published in 2012. The new version, VITAL 2.0, is based on the VITAL Scientific Expert Panel’s (VSEP) comprehensive assessment of the latest scientific data on food allergies. Updates to the state-of the art are intended to substantiate the significance of the tool for allergen management and risk assessment in the food industry. The tool was introduced in 2007. A number of renowned companies in Australia and New Zealand are already using it to help them label possible allergen traces in foods clearly and consistently.
The most important changes published before this final launch are summarised below.
Action Levels
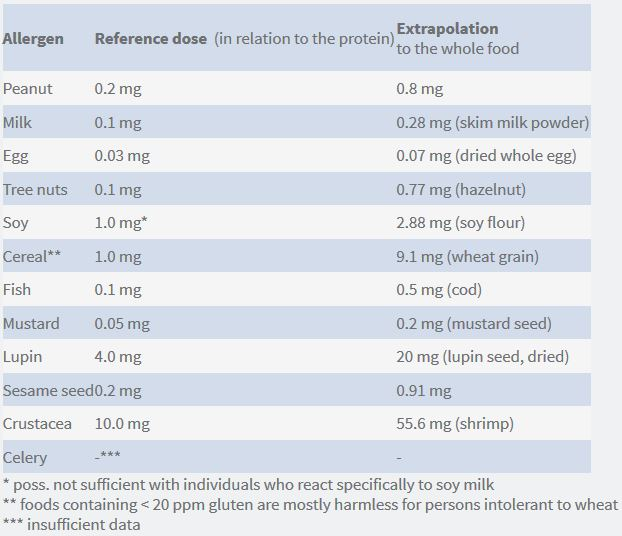
The three-stage VITAL grid with threshold values for Action Levels 1 – 3 that has been existing so far has been abolished. Instead, an interactive grid was introduced. It determines variable Action Levels for the different allergens based on the respective reference dose and the product-specific reference amount. The reference dose specifies the total amount (in mg) of protein from an allergenic food, below which only extremely sensitive allergic persons (1 – 5 %) will experience an adverse reaction (see table). The reference amount or serving size is defined as the maximum amount of a product that is consumed in a typical eating occasion. It is the food manufacturer’s task to define this amount for each relevant product.
Along with this, there will no longer be an Action Level that triggers ingredient labelling (“Contains XY”) – in the event of heavy cross-contaminations, manufacturers are encouraged to assume responsibility and minimise the risk accordingly.
Click on the image below to view the list of VITAL reference doses:
Gluten
As allergy sufferers may be affected not only by the gluten fraction but also by the other cereal-derived proteins, the Vital concept takes into account not only the presence of gluten but also the total protein quantity present in the cereal when determining the portion size limit values. While the relevant action level varies - as in the case of the other allergens - depending on the portion size, they are capped at 20 ppm to avoid conflict with the statutory requirements for the marking of gluten-free products.
Your Vital 2.0 Trainer
| Dr. Martin Röder |
Dr. Wolfgang Weber |
 |
 |