Monochloro propandiole (MCPD), glycidol and their fatty acid esters
Monochloro propandiole 3-monochloro-1,2-propandiol (3-MCPD) and 2-monochloro-1,3-propandiol (2-MCPD) are part of the group of process contaminants and are primarily produced in the manufacture of hydrolysed vegetable protein and soy sauce. Toasting, baking, frying and roasting processes can also induce the formation of 3-MCPD and 2-MCPD in, for example, bread, meat, fish and coffee.
Structural formulae of 3-monochloro-1, 2-propandiol (left) and glycidol (right):
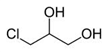
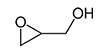
3-MCPD fatty acids esters and glycidyl fatty acid esters in foodstuffs
In addition to free 3-MCPD, 3-MCPD fatty acid esters and glycidyl fatty acid esters were found in various edible vegetable fats and oils - primarily palm oil - for the first time in 2006. Due to the processing of edible vegetable fats and oils, 3-MCPD fatty acid esters and glycidyl fatty acid esters can occur in increased concentrations in the following foodstuffs in particular:
- Infant and follow-on formula
- Hazelnut spreads
- Margarine and other fatty spreads
- Fried food (e.g. fish fingers)
- Pastry products
How do 3-MCPD fatty acid esters and glycidyl fatty acid esters develop?

3-MCPD fatty acid esters and glycidyl fatty acid esters primarily develop during fat and oil refining. Refining is a necessary chemical and physical filtration and purification process to ensure the quality of fats and oils. One element of the fat refining process is deodorisation and deacidification, whereby unwanted odorous and flavouring substances as well as free fatty acids are removed by pressure and heat treatment. The heat used during this process can cause the monoacylglycerides (MAG), diacylglycerides (DAG) and triacylglycerides (TAG) to react with the chloride compounds naturally occurring in the oils and fats to form 3-MCPD fatty acid esters and glycidyl fatty acid esters. 3-MCPD fatty acid esters usually form at temperatures around 180°C. Temperatures above 230°C promote the development of glycidyl fatty acid esters.
How do 3-MCPD fatty acid esters and glycidyl fatty acid esters develop?
Not all vegetable oils are affected in the same way. Some seed oils such as rapeseed, soya and sunflower oil contain levels of 3-MCPD fatty acid esters and glycidyl fatty acid esters below the detection limit, whereas fruit oils, such as refined palm or olive oil, contain much higher levels. However, because of their specific properties, it is not a matter of simply replacing fruit oils with seed oils.
1-stearoyl-3-MCPD (example of a monoester):
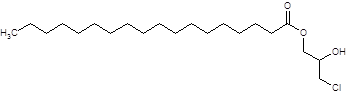
1-palmitoyl-2-oleoyl-3-MCPD (example of a diester):
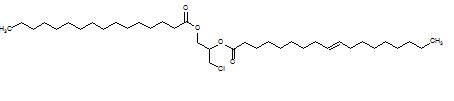
What are the risks posed by 3-MCPD, 2-MCPD and their fatty acid esters?
Laboratory tests on animals have shown that long-term increased ingestion of free 3-MCPD can cause excessive cell formation (hyperplasia) in the renal tubuli. In 2012 the International Agency for Research on Cancer (IARC) deemed 3-MCPD ‘potentially carcinogenic to humans’ (IARC category 2B). In March 2016 a panel of experts from the European Food Safety Authority (EFSA) evaluated the potential risk from 3-MCPD. Their publication confirmed that ester-bound 3-MCPD is almost completely split by the human digestive system. This means that the risk from 3-MCPD fatty acid esters is almost identical to that from free 3-MCPD. The EFSA updated its assessment for 3-MCPD in November 2017 and deduced a TDI of 2.0 µg/kg body weight for 3-MCPD and its fatty acid esters.
Sample calculation
An adult with a body weight of 60 kg should not ingest more than 120 µg 3-MCPD and its fatty acid esters per day (2.0 µg × 60 kg). Consuming just 50 g of hazelnut spread, for example, which contains 4.0 mg/kg 3-MCPD fatty acid esters (expressed as 3-MCPD), already significantly exceeds the TDI value of 2.0 µg/kg body weight (50 g × 4 mg/kg = 150 µg).
According to the EFSA the data currently available is insufficient to assess the risk of 2-MCPD and its fatty acid esters. Thus there is no evaluation at present, however one is planned for the future.
What risks are posed by glycidyl fatty acid esters?
In 2012 the IARC deemed 2,3-epoxy-1-propanol (glycidol) to be ‘probably carcinogenic to humans’ (IARC category 2A), and it is considered a genotoxic carcinogen. In March 2016 the EFSA also assessed the potential risk from glycidyl fatty acid esters. As with 3-MCPD fatty acid esters, ester-bound glycidol is almost completely released by the human digestive system. The toxicology of glycidyl fatty acid esters is evaluated using the MoE (Margin of Exposure) approach. Further information about the toxicological evaluation of glycidyl fatty acid esters can be found in the BfR’s Notice no. 020/2020 of 20 April 2020.
What are the legal stipulations?
At present Ordinance (EC) no. 1881/2006 stipulates maximum levels for ‘free’ 3-MCPD and glycidyl fatty acid esters, expressed as glycidol.
There are currently no maximum levels for 3-MCPD fatty acid esters. However, the European Commission is planning to set maximum levels for the total of free 3-MCPD and its esters by 2021 at the latest.
Analytics by the ifp Institute for Product Quality
We determine 3-MCPD fatty acid esters (expressed as 3-MCPD) and glycidyl fatty acid esters (expressed as glycidol) using the officially validated unit method DGF C-VI 18 (10) A, B, also known as AOCS Cd 29c-13.

