Water for Pharmaceutical Use (WPU)
Due to the significance of processed water in the pharmaceutical industry, either as a product ingredient or as water used in the production process, the quality requirements regarding this important raw material are especially high.
The purity criteria are defined in the monographs of the European Pharmacopoeia (Ph. Eur.) (monographs acc. to Ph. Eur., in current version).
- Purified water (Aqua purificata) is intended for the manufacture of drugs that are not required to be sterile nor pyrogen-free. It is divided into “Purified water in bulk” and “Purified water filled into containers”.
- Highly purified water (aqua valde purificata) is designed for the manufacture of drugs that require the use of water that is of high biological quality.
- Water for injection (aqua ad iniectabilia) is intended for the manufacture of drugs for parenteral use whose solvent is water. There is “Water for injection in bulk“ and “Sterilised water for injection”.
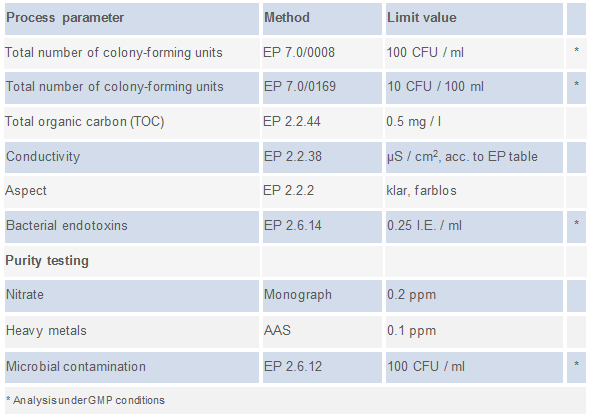
ifp Institut für Produktqualität offers quality checks based on the procedures to be applied according to Ph. Eur. as described below (click on the image below to see the list of methods):